L.DOT Study Management software
Managing the workflow and privacy compliancy for medical research studies is challenging. The duration of the study, staff involved and strict privacy / security regulations are an administrative challenge. With L.DOT Study Workflow software this burden will be greatly minimized.
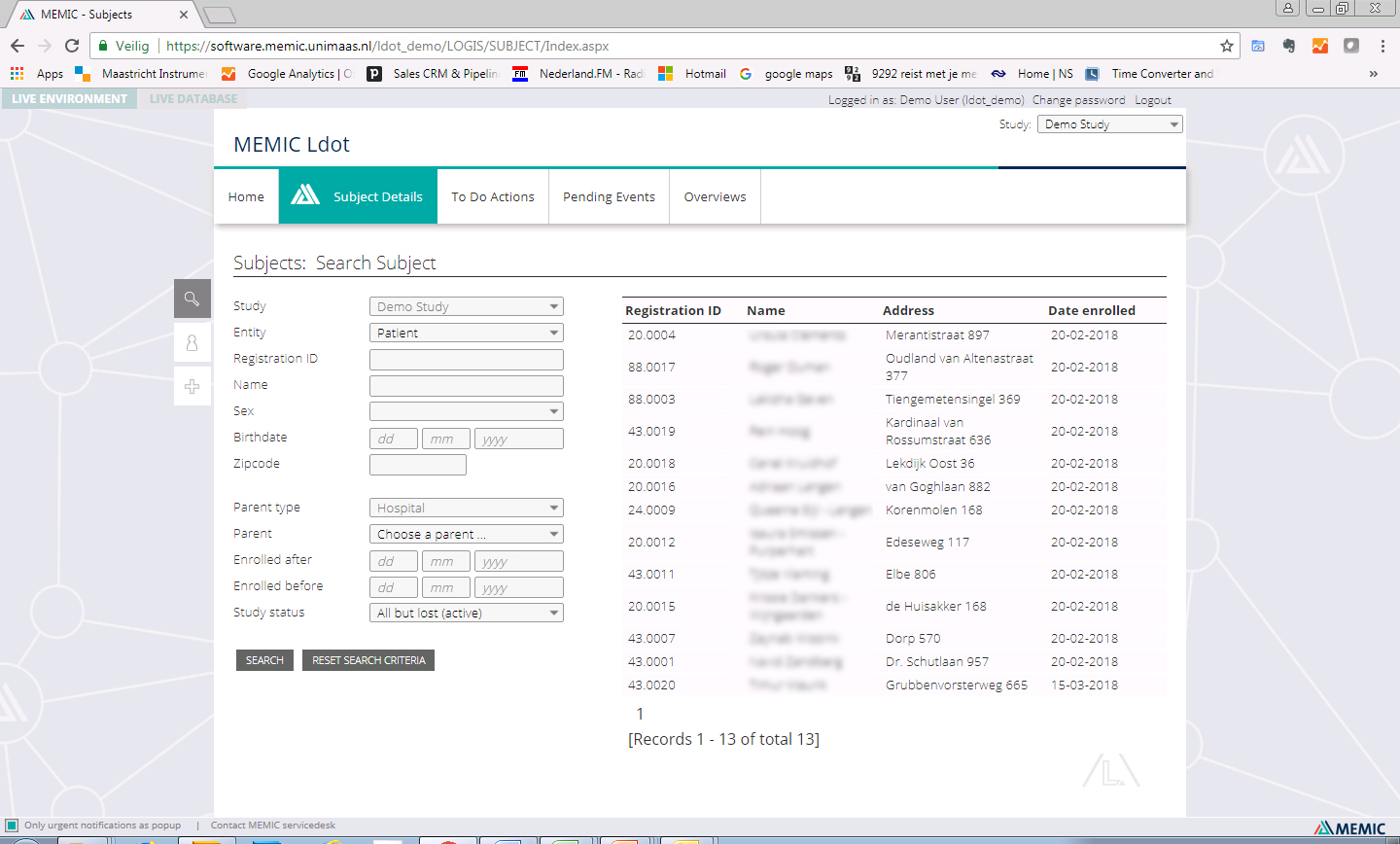
- Use L.DOT to make your study privacy compliant and separate the personal and logistics data from study data. Reduce the risk of data leaks for participant data.
- Maintain full control over execution of the research plan, independent of the experience and skills of staff involved in the study.
- L.DOT can be used in studies of any size, also for multi-centre trials, independent of the type of electronic data capture systems used in the study.
- L.DOT is trusted in dozens of studies for more than a decade, and is now available for research teams worldwide.
Key features

Save time and money in executing your study

Safeguard all personal data required to contact participants

Secure access for multi-centre collaborations

Register the logistic trail of your obtained research data

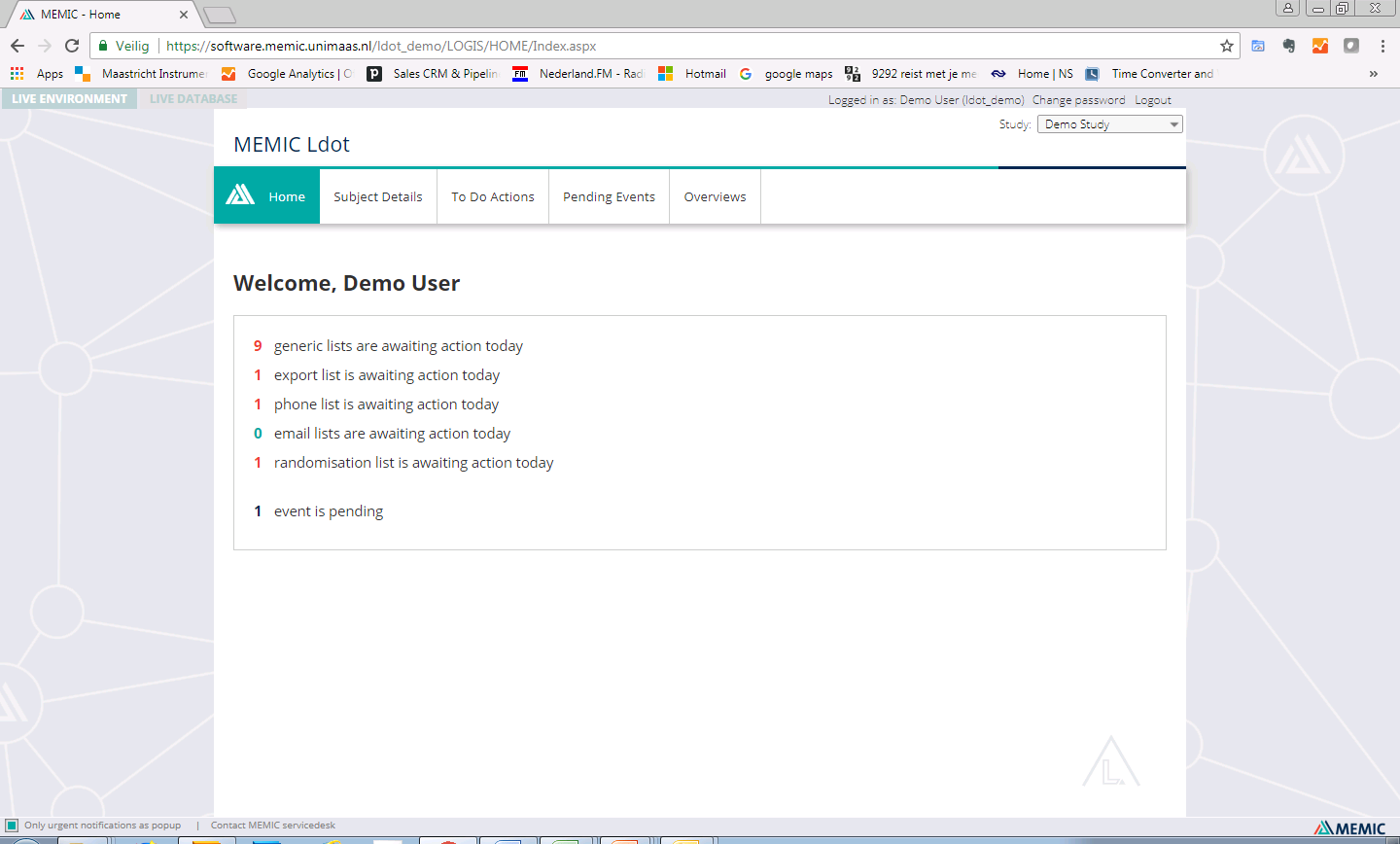
Keep track of required actions for each participant

Ensure standardized execution of the research protocol
Product specifications
L.DOT is a web-based tool, designed to monitor the study logistics and guard the progress of your research project. It supports the study workflow by indicating which actions should be performed for which study subject at what time. L.DOT can be configured to present this information as email notification, action list or in a graphical calendar. L.DOT is designed specifically for protocols that require safe storage of personal data and recommended for studies with a larger number of participants, a more complex or longitudinal schedule and multicentre studies.
- The web application only stores personal and logistical data and not research data.
- No patient data is stored in Ldot (data can be stored in another EDC tool)
- Ldot conforms to the European data protection act, the General Data Protection Regulation (GDPR)
L.DOT features
| Security & regulatory compliancy | ||
| GCP compliant | ✓ | |
| GDPR compliant | ✓ | |
| Daily backups – daily | ✓ | |
| Data backups – hourly | ✓ | |
| Operating platform | Online – internet explorer / firefox / chrome | |
| Participant management | ||
| Subject management (add, view, search, edit) | ✓ | |
| Email integration (templates) | ✓ | |
| SMS integration | ✓ | |
| Randomisation | ✓ | |
| User management | ||
| User management (roles & access management) | ✓ | |
| Multi-centre user management | ✓ | |
| Study management | ||
| Study management (add study, view study, search studies, edit study) | ✓ | |
| Standardization of study protocol | ✓ | |
| Alerts for actions that require attention | ✓ | |
| Automatic execution of protocol rules | ✓ | |
| Automatic execution of specific actions | ✓ | |
| Automatic registration by third party applications | ✓ | |
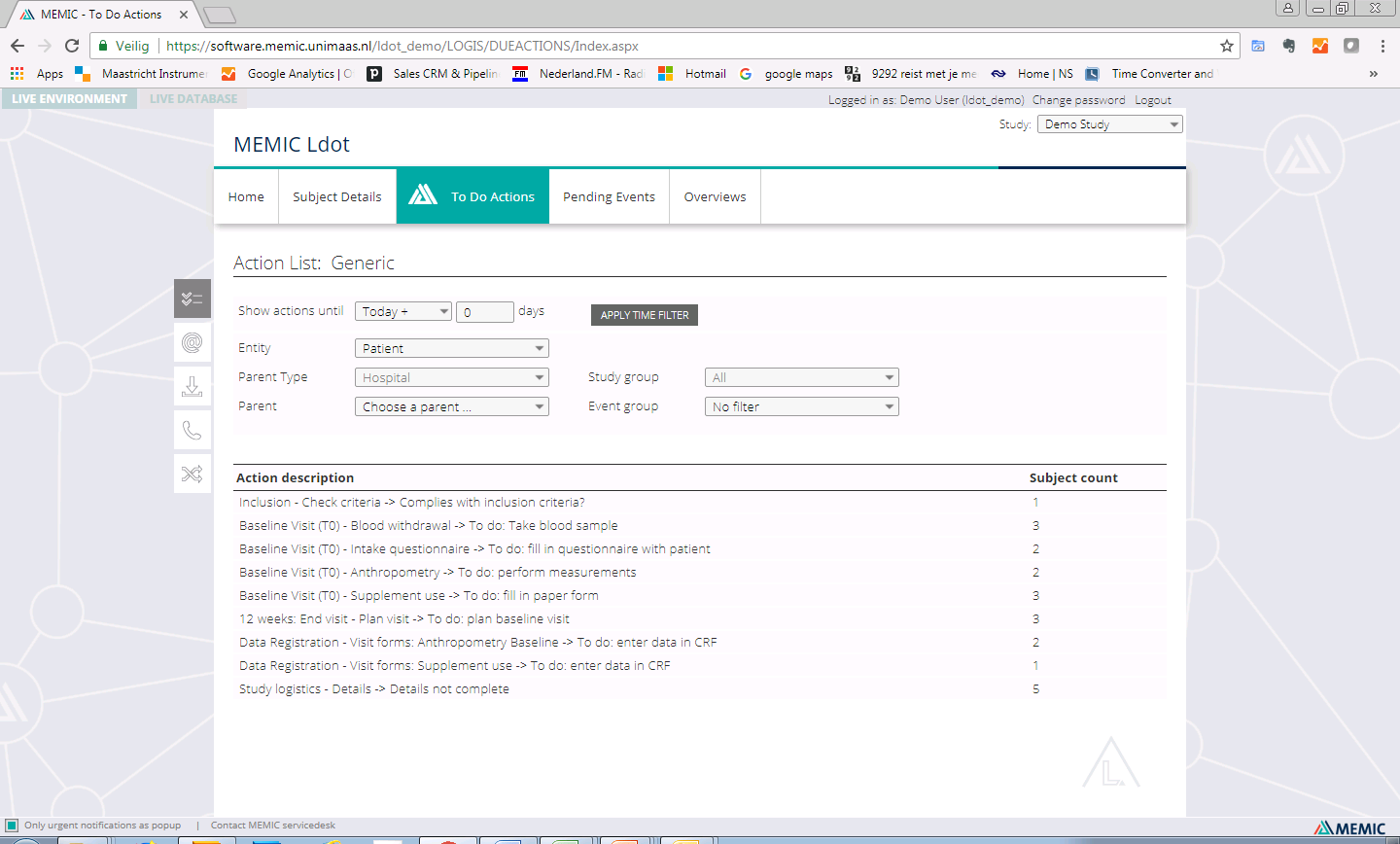
| To-do actions (list view) | ✓ | |
| To-do actions (calendar view) | ✓ | |
| To-do actions (generic actions) | ✓ | |
| To-do actions (email list) | ✓ | |
| To-do actions (export list) | ✓ | |
| To-do actions (phone list) | ✓ | |
| To-do actions (randomisation list) | ✓ | |
| Registration of LOF – loss to follow up (three levels PreLOF / Exclusion / LOF) | ✓ | |
| Study reporting | ||
| Subject loss reasons (PreLOF / exclusion / LOF) | ✓ | |
| Subject status (x days / x months / complete) | ✓ | |
| 3rd party data collection integration | ||
| MOXNET | ✓ | |
| Qualtrics | ✓ | |
| Castor EDC | ✓ | |
| OpenClinica | ✓ | |
| Backend features (not visible for client, for management purposes only) | ||
| User management – system administrator features | ✓ |
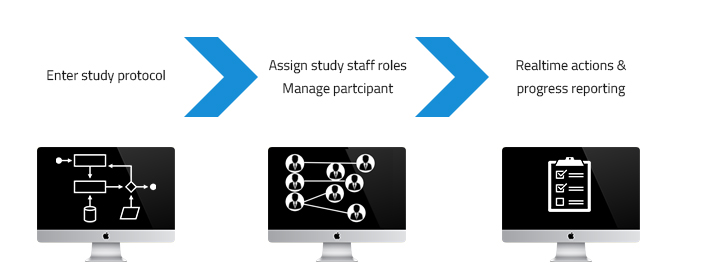
How it works
Testimonials

A multicenter, investigator initiated, prospective cohort study as EnCoRe can’t do without the Ldot infrastructure.
Prof. dr. ir. Matty P. Weijenberg

A work flow support tool like Ldot ensures that our participants receive the right message at the right time.
Lucy Overbeek, Project Manager Healthy Brain Study Radboud UMC
Request a quote
Address and contact info
Maastricht Instruments BV
Universiteitssingel 50
6229 ER Maastricht
+31 (0)43-3881371
email@maastrichtinstruments.com